Articles from RevBio, Inc.

RevBio® announced today that TETRANITE, the company’s first of its kind regenerative “bone glue” has been approved for a 20-patient pilot study to measure the biomaterial’s safety and efficacy to treat complex, multi-fragmented wrist fractures including those that involve articular surfaces.
By RevBio, Inc. · Via Business Wire · November 4, 2025

RevBio, Inc., announced that it has been awarded a $2.2 million Phase II Small Business Innovation Research (SBIR) grant from the National Institute on Aging (NIA), part of the National Institutes of Health (NIH). This two-year grant (1R44AG097243-01) builds upon the work that was completed in a prior Phase I grant (1R43AG079741-01A1) and will enable the company to complete preclinical product testing which is a key development step for obtaining approval from the U.S. Food & Drug Administration to initiate a human clinical trial.
By RevBio, Inc. · Via Business Wire · October 6, 2025

RevBio, Inc., announced that it has received approval from the Medicines and Health Regulatory Agency (MHRA) to conduct a 15 patient pilot clinical trial to use TETRANITE® to reintegrate bone flaps, which are portions of the skull that are temporarily removed by neurosurgeons to perform brain surgery. This clinical trial is designed to demonstrate that TETRANITE can reintegrate these bone flaps with the surrounding bone to improve cosmesis, increase flap stability for patient comfort, and help prevent cerebrospinal fluid leaks, which can cause significant pain and lead to serious infections.
By RevBio, Inc. · Via Business Wire · May 29, 2025

RevBio, Inc., announced that it has received regulatory and ethics committee approvals in multiple European countries to conduct its pivotal clinical trial for its dental implant stabilization product. The successful completion of this pivotal clinical trial will result in the CE marking approval for the product, which will allow the company to begin commercial sales in Europe. As of the date of this press release, the company has already enrolled 30 of an expected 75 patients in this clinical trial.
By RevBio, Inc. · Via Business Wire · March 17, 2025

The United States Patent and Trademark Office recently issued patent 12,178,937 entitled “Compositions and Methods for Adhesion to Surfaces,” which constitutes the 10th U.S. patent that covers the TETRANITE bone adhesive technology. This patent expands the protected technology to include injectable mineral-organic structural bone adhesive compositions that comprise alpha tricalcium phosphate. The issuance of this patent complements RevBio’s existing U.S. patent 11,638,777, which covers the method of repairing fractured bone using the aforementioned adhesive composition. These recently issued patents enlarge RevBio’s portfolio of adhesive compositions for bone repair. The novel TETRANITE biomaterial is also the only patented bone adhesive to include phosphoserine, an organic compound, which has been shown in published literature to play a role in the process of bone regeneration.
By RevBio, Inc. · Via Business Wire · January 30, 2025
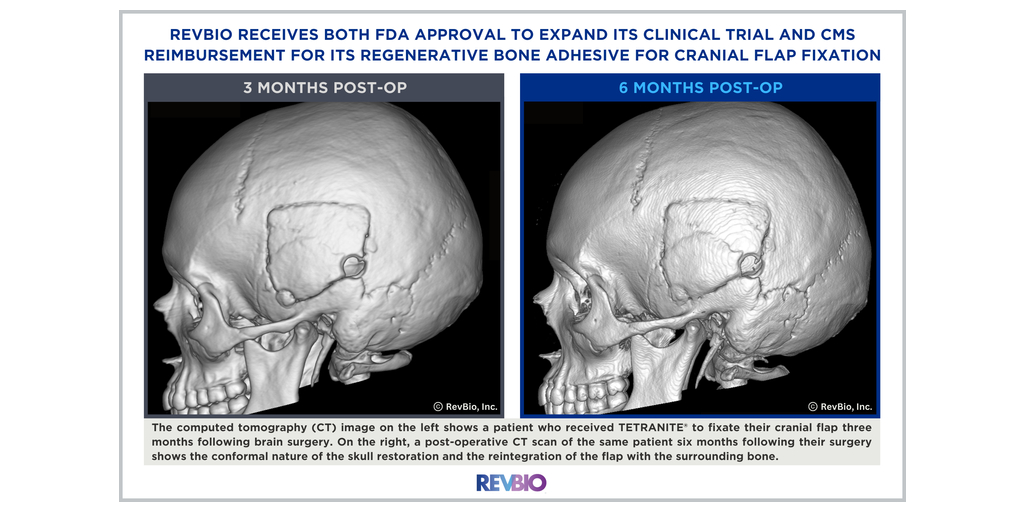
RevBio, Inc., announced that it has received FDA approval to expand its ongoing clinical trial to immediately fixate cranial flaps using TETRANITE®, the company’s bone adhesive biomaterial. The company also received reimbursement coverage from the Centers for Medicare and Medicaid (“CMS”) for the use of TETRANITE to replace metal plates and screws.
By RevBio, Inc. · Via Business Wire · December 11, 2024
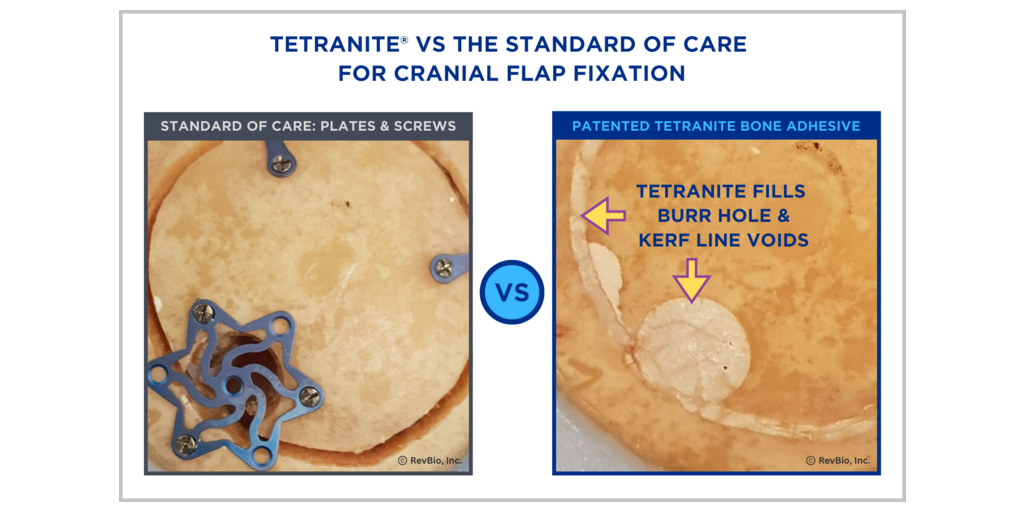
RevBio, Inc., announced that it has received approval from the U.S. Food and Drug Administration to start a 20-patient pilot clinical trial to examine the safety and efficacy of the company’s bone adhesive biomaterial called TETRANITE® to immediately stabilize and fixate cranial flaps following craniotomy procedures associated with brain surgery. This approval was largely predicated on the successful pivotal preclinical and surgeon handling testing conducted by the company.
By RevBio, Inc. · Via Business Wire · December 8, 2023

RevBio, Inc., announced that it has been awarded a $2.4 million Phase II Small Business Innovation Research (SBIR) grant from the National Institute of Neurological Disease and Stroke (NINDS), part of the National Institutes of Health (NIH). This two-year grant (1R44NS135736-01A1) will allow the company to complete a 20-patient pilot clinical trial to examine the safety and efficacy of the company’s bone adhesive biomaterial called TETRANITE® which will be used to immediately fixate cranial flaps and enable bone fusion following craniotomy procedures associated with brain surgery.
By RevBio, Inc. · Via Business Wire · September 16, 2024

RevBio, Inc., announced that it has received a grant from the National Institutes of Health through its Helping to End Addiction Long-term Initiative, or NIH HEAL Initiative, to improve strategies for the prevention and treatment of opioid misuse and addiction. This $2 million Phase II Small Business Innovation Research (SBIR) grant (2R44DE029369-02) will fund the pre-clinical development of a dental bone graft formulation that will include the release of locally acting non-opioid pain medication. This product is intended to be used to fill extraction sites and mitigate post-operative pain following the removal of wisdom teeth.
By RevBio, Inc. · Via Business Wire · October 25, 2023
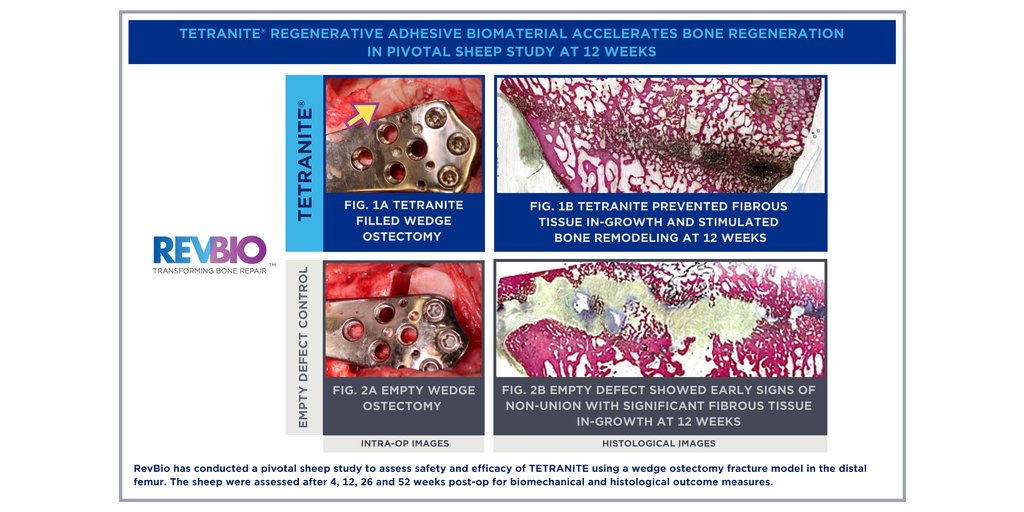
RevBio, Inc., announced that it has been awarded a grant from the National Institute on Aging (NIA), part of the National Institutes of Health (NIH), through its Commercialization Readiness Program of up to $3.4 million over the next three years (1SB1AG085803-01). This grant will enable the company to finish its late-stage product development activities and conduct a clinical trial to demonstrate the safety and efficacy of the product in comparison to the current standard of care.
By RevBio, Inc. · Via Business Wire · October 6, 2023
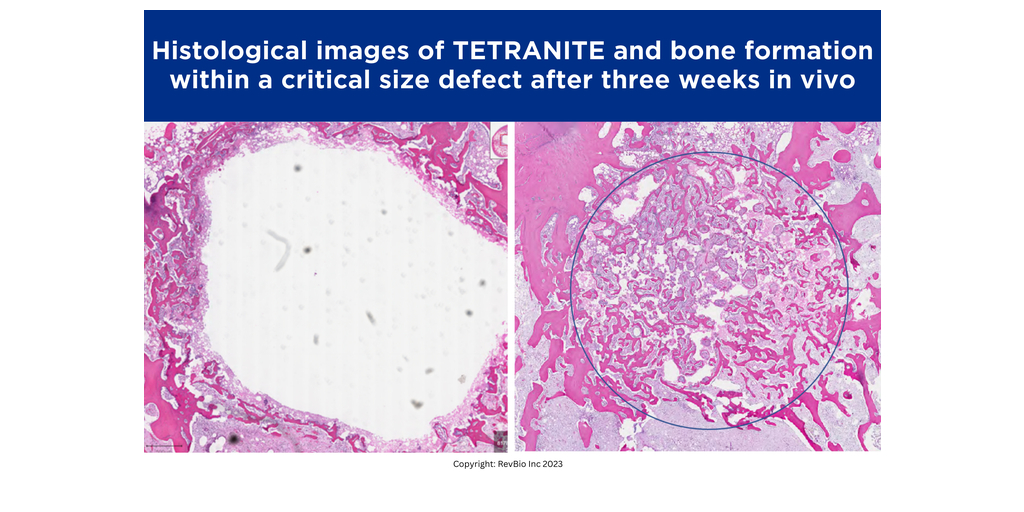
RevBio, Inc., announced that it has received approval from the U.S. Food and Drug Administration to start a 20-patient clinical trial to examine the safety and efficacy of a more rapidly replaced, pH modified porous formulation of the company’s bone adhesive biomaterial called Tetranite® to immediately stabilize dental implants following tooth extractions. This new formulation has shown evidence of a more biologically active bone substitution. While not osteoinductive, this patent-pending version of Tetranite has shown characteristics which may be described as “osteopromotive.”
By RevBio, Inc. · Via Business Wire · August 2, 2023

RevBio, Inc., announced that it has been awarded a $2 million Phase II Small Business Innovation Research (SBIR) grant from the National Institute of Dental and Craniofacial Research (NIDCR), part of the National Institutes of Health (NIH). This funding (1R44DE032564-01) will allow the company to complete the pre-clinical research necessary to advance this product into the clinical stage of development.
By RevBio, Inc. · Via Business Wire · June 7, 2023

RevBio, Inc., announced that it has received ISO 13485 certification for its quality management system. Receiving ISO 13485 certification indicates that a company has developed robust policies and procedures for the development and manufacture of regulated medical products.
By RevBio, Inc. · Via Business Wire · May 22, 2023

RevBio, Inc. announced that it has received a strategic investment from Pacific Dental Services® (PDS) to support the clinical development of its bone adhesive biomaterial for implant dentistry. Based in Irvine, California, PDS was founded in 1994 and is one of the leading dental support organizations in the U.S. with over 900 dental practices in 25 states. PDS has been on the Inc. 5000 list of the fastest growing private companies in America 14 times.
By RevBio, Inc. · Via Business Wire · February 14, 2023
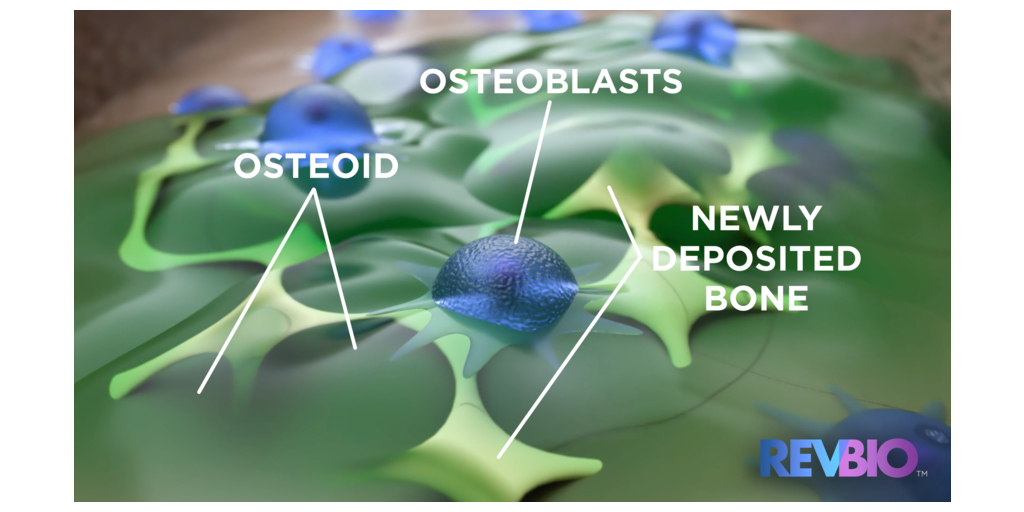
RevBio, Inc., announced that it has received approval from the Italian Ministry of Health and the Ethics Committee of the University “G.D'Annunzio” of Chieti-Pescara to start a 15-patient pilot clinical trial. The primary objective of this study will be to assess the time it takes for Tetranite®, the company’s adhesive biomaterial, to regenerate bone in the mandibular and maxillary dental arches.
By RevBio, Inc. · Via Business Wire · January 18, 2023

RevBio, Inc., announced that an experiment to study Tetranite®, the company’s regenerative bone adhesive biomaterial, has successfully been initiated onboard the International Space Station (ISS). On Saturday, November 26, 2022, the study materials were launched to the space station on SpaceX’s 26th Commercial Resupply Services (SpaceX CRS-26) mission, sponsored by the ISS National Laboratory. This in vivo research, which will be conducted over the next two months on the ISS, will examine the biomaterial’s ability to regenerate bone when used in a microgravity environment where bone growing conditions and the ability to regenerate new bone tissue is significantly compromised.
By RevBio, Inc. · Via Business Wire · December 9, 2022
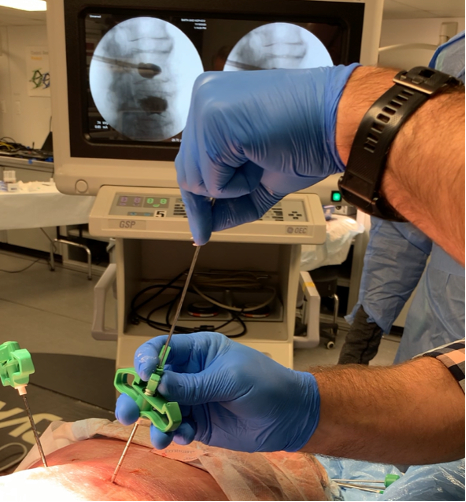
RevBio, Inc., announced that it has been awarded a Phase I Small Business Innovation Research (SBIR) grant from the National Institute on Aging, part of the National Institutes of Health. This funding will allow the company to pursue the treatment of vertebral compression fractures with its patented bone adhesive technology known as Tetranite®. Because the osteoconductive bone adhesive is injectable, the material may be delivered in a minimally invasive procedure.
By RevBio, Inc. · Via Business Wire · September 20, 2022

RevBio, Inc., announced that it has received approval from the Medicines and Healthcare products Regulatory Agency in the United Kingdom to start a 15-patient clinical trial to examine the safety and efficacy of immediately stabilized dental implants following tooth extractions using an optimized formulation of Tetranite®, the company’s bone adhesive biomaterial.
By RevBio, Inc. · Via Business Wire · September 15, 2022
